Abstract
Here is proposed a new hypothesis where acidity, evoked by stress, has an important role in the generator mechanism of atherosclerotic lesions, giving a new perspective for the understanding of its etiology and pathogenesis. The acidity theory of atherosclerosis is inside the response to injury concept.
It has the following sequence of events:
I. Sympathetic dominance by continuous stress plus
II. Deficiency in production of endogenous digitalis-like compounds (DLCs) with alterations of Na(+), K(+)-ATPase activity results in:
III. Lowered pH (acidity) that increases perfusion pressure and provokes effects on contractility of coronary arteries leading to changes in hemodynamic shear stress and atherosclerosis as consequence. The heart is an organ of high metabolic activity, susceptible to drops in pH during ischemia and hypoxia. Chronic elevated sympathetic bias may accelerate the myocardial anaerobic glycolysis with a significant increase in lactate production. In hypertension the concentration of lactic acid in both venous and arterial blood may be significantly elevated.
Lactic acid in blood plasma is also significantly elevated during stress situations and indicative of stress levels. Psychosocial factors are independent significant predictors of carotid intima-media thickness (IMT) progression. Stress reduction through behavioral changes or use of
sympatho-inhibitory drugs like Beta-blockers slow the progression of carotid IMT. Cardiac glycosides at lower daily doses also blocks excessive catecholamine release, resulting in very low mortality rate in prevention of acute coronary syndromes in patients with heart disease, as treated under the myogenic theory of myocardial infarction, a complementary hypothesis. Cardiac glycoside drugs show additional therapeutic possibilities, like re-elevation of lowered pH, appearing to attend the demand in insufficient production of endogenous DLCs, in some clinical conditions.
“Certainly all tissues change with age. There is anatomic and chemical aging. The acidity of
tissues increases with age; this favors the precipitation of cholesterolâ€, O. J. Pollak, 1952 (1) 2
The Hypothesis
The present hypothesis follows the response to injury concept of atherosclerosis developed by
Russel Ross, John Glomset and Laurence Harker in 1977 (2). According to this concept,
physiologically active substances are released in response to injury of the arterial endothelium,
and these substances induce pathologic reactions by the cells constituting the vascular wall.
Our hypothesis was developed in the mid 2006 (3), inspired by the demonstration that normal
stretching/relaxing of an artery does not produce atherosclerosis, while stretching/relaxing in
different directions simultaneously on every heart beat does. This discovery from scientists in
California (4, 5, 6) prompted us to search for other potential mechanisms beyond the simplistic
idea of cholesterol as the culprit, and could offer:
1) An alternative understanding for the etiology and pathogenesis of atherosclerosis
2) A compatible and efficient therapy according the mechanism in question
3) To be suitable with the myogenic theory of myocardial infarction, developed by Quintiliano H. de Mesquita in 1972 (7, 8), which we support since that time. The myogenic theory accepts
atherosclerosis as responsible for the reduced regional myocardial function, relationship
recognized by participants of the MESA study in a paper published in 2006 (9).
We believe the Acidity Theory of Atherosclerosis attend these premises.
The sequence of events according our proposition:
I. Sympathetic dominance by continuous stress plus,
II. Deficiency in production of endogenous digitalis-like compounds with alterations of Na(+),
K(+)-ATPase activity results in,
III. Lowered pH (acidity) that increases perfusion pressure and provokes effects on contractility
of coronary arteries, leading to changes in hemodynamic shear stress and atherosclerosis as
consequence.
The acidity theory of atherosclerosis does not underestimate the importance of other key factors
for atherosclerosis like ageing, improper diet, environmental pollution, lifestyle, physical
inactivity, tobacco smoking and genetic predisposition. However, most of these risk factors
might result in altered autonomic nervous system, sympathetic bias, increased lactic acid and
acidic environment thus propitiating atherogenesis. Our proposal may extend to any respiratory
or metabolic disturbances resulting in acidosis.
Note: We prefer to use the term coronary-myocardial disease rather coronary artery disease or coronary heart disease, to make clear the dual pathologies involved, according the theories we defend.
Evidence and Fundamentals
Coincidentally, a very recent paper attempted to explain the mechanism behind the association
of many disparate risk factors like diet, age, gender, family history, stress, lifestyle, smoking,
diabetes, dyslipidemias, hypertension and HIV, believing these could encourage the
development of atherosclerosis by inducing adventitial autonomic dysfunction and sympathetic
bias. Accordingly, the authors have proposed that atherosclerosis is caused by stress
dysfunction particularly of neurogenic origin (10).
Studies linking stress to atherosclerosis are not new. We should remember that Hans Selye
proposed in 1950 that stress could induce hormonal autonomic responses and, overtime, these
hormonal changes lead to atherosclerosis and other diseases (11). Walter Cannon was the first
to demonstrate, in 1914, that acute stress results in increased outpouring of adrenaline (12).
Many cardiovascular disease processes, including myocardial ischemia, congestive heart
failure, unstable angina pectoris, acute myocardial infarction, heart broken syndrome,
arrhythmias and ischemic stroke are precipitated or worsened by perturbations in the autonomic nervous system, with sympathetic activation and excessive secretion of catecholamine (adrenaline and noradrenaline) (13-23). 3
Even hypertension, traditionally considered as originated from kidneys, now is regarded as
triggered primarily through the nervous system and later exacerbated by non-neural factors (24).
Indeed, the use of sympatholytic agents (55) and stress reduction approaches (25) may lead to a
significant reduction in blood pressure levels.
Related to mental stress and atherosclerosis, studies have showed that:
a) Higher rise in systolic blood pressure during psychological stress results in a more severe
and greater progression of carotid atherosclerosis (26, 27, 28);
b) Adrenaline and noradrenaline may act as chemical mediators during atherogenesis in man,
thus contributing to the development and subsequent complications of atherosclerosis (29);
c) Mental stress – induced pulse pressure changes may influence the development of early
atherosclerosis in the carotid artery of woman (30);
d) Blood pressure changes during psychological stress predict subsequent coronary
calcification in young health adults 13 years later (31);
e) Brief episodes of mental stress, similar to those encountered in everyday life may cause
transient (up to 4 hours) endothelial dysfunction in healthy young individuals (32);
f) Delayed blood pressure recovery after psychological stress is associated with carotid intimamedia thickness (IMT) (33);
g) Psychosocial factors are independent significant predictors of IMT progression (34);
h) Depressive symptoms are associated with the development of atherosclerosis (35).
Deficiency of endogenous digitalis-like compounds, the sodium potassium pump and
cardiac glycosides Endogenous digitalis-like compounds (DLCs) of the cardenolide (digoxin and ouabain / strophanthin) and bufadienolide (Proscillaridin-A and Marinobufagenin) types, recently isolated from human tissues and body fluids, have similar molecular structure of cardiac glycosides extracted from plants and toad venom (36, 37). Endogenous DLCs are steroidal hormones that are synthesized in, and released from the adrenal gland, whose regulation may be directed by the hypothalamic-pituitary-adrenal (HPA) axis (38, 39).
Cholesterol, a vital substance produced by the human body, is the major precursor of endogenous digitalis-like compounds (40).
Many hormones, including aldosterone, insulin, thyroid hormone and catecholamines regulate
not only the expression but also the insertion of Na+, K+-ATPase into the plasma membrane,
according to specific physiological needs. The Na+, K+-ATPase which was considered the ion
transporting pump now appears to have many other unrelated functions, some of which may be
regulated by DLC. In fact DLCs have already been implicated in the regulation of several major
physiological parameters including water and salt homeostasis (37).
In many cases, perturbation of the DLC system has been implied in pathological conditions
including cardiac arrhythmias, hypertension, cancer and depressive disorders (37, 41).
Stress situations may affect the release of endogenous digitalis-like compounds by the adrenal
gland (39). Also, the extracellular acidification may affect the signaling and transport of
endogenous DLCs (42, 43). This raises the possibility that an insufficient production of
endogenous DLCs to attend the demand in some medical conditions, like coronary-myocardial
disease, hypothetically can be resolved through the use of cardiac glycosides at low
concentration, as a supplement. This postulation is confirmed by clinical studies using cardiac
glycosides with largely positive effects in prevention of acute coronary syndromes (44, 45, 46).
Cardiac glycosides, that in higher concentrations inhibit the Na/K pump, in low therapeutic
doses (147), including at nanomolar range concentration (47), can stimulate it. Also, cardiac
glycosides have specific sympathoinhibitory response by blocking the overproduction of
catecholamine. This property is unrelated to the positive inotropic action of cardiac glycosides
(48, 49). We hypothesize that endogenous digitalis-like compounds may have similar action on
neurohormonal levels.
It has been shown that cardiac glycosides can re-elevate lowered pH by stopping the over
production of lactic acid by the heart and in some other clinical and experimental conditions (50, 451, 52, 53). It is interesting to note that studies have suggested a causal relationship between noradrenaline/adrenaline and concentrations of lactic acid (54).
We should also notice the cardiac glycosides digoxin and digitoxin may lower blood pressure in
hypertensive patients (55). Paradoxically, some studies have shown that infusion of ouabain
over several weeks produces hypertension in rats (56), situation in which digoxin and digitoxin
can also lower the blood pressure (57). There was no evidence of ventricular hypertrophy in
animals receiving ouabain in this study, despite the documented hypertension, with the authors
considering that ouabain may actually be cardioprotective (56).
It was recently demonstrated that cardiac glycoside drugs potently block activation of NF-kB
signaling pathway, providing a feasible therapeutic use for the treatment of inflammatory
diseases, like in atherosclerosis (58). The activation of Nuclear factor-kappa B (NF-kB), that has
been called a “smoke sensor†of the body, is induced by a variety of agents including stress,
cigarette smoke, viruses, bacteria, inflammatory stimuli, cytokines, free radicals, carcinogens,
tumor promoters, and endotoxins.
We think a deficient production of DLCs may result in a dysfunctional HPA axis response
associated with increased susceptibility to inflammatory disease (59).
A recent study has identified phagocytes (macrophages and neutrophils) as a new source of
catecholamines, which may enhance the inflammatory response (146).
Acidic environment and cardiovascular diseases
The heart is an organ of high metabolic activity – that cannot rest as other body muscles, being
susceptible to drops in pH during ischemia and hypoxia (13). The chronic or acute elevated
catecholamine release, mainly from sympathetic nerve terminals in cardiac tissue (60), with
alterations in Na(+), K(+)-ATPase activity, may accelerate the myocardial anaerobic glycolysis
leading to significant increase in lactate production.
Studies have shown that:
a) Either in essential or renal hypertension the concentration of lactic acid in both venous and
arterial blood may be significantly elevated (61)
b) Lactic acid in the blood plasma is significantly elevated during stress situations and serving
as indicative of stress levels (62, 33);
c) Catecholamines may be important determinants for the development of ketoacidosis and/or
lactic acid (54).
d) Lowered pH increases perfusion pressure (64, 65). Also, pH changes have profound effects on
contractility of coronary arteries (65, 66), that may happens through the sodium/potassium pump and K induced relaxation channels (67);
e) Lactate, lowered pH and lactic acid induce endocardial damage (68);
f) A decreased pH may be associated with an increased blood-pressure response to salt
loading (69);
g) Ingestion of glucose, fructose and other sugars may have the effect to raise blood lactic acid
with this increase being most marked and lasting longest after fructose, that is largely used as
sweetener in soft drinks, fruit punches, pastries and processed foods. Dietary fructose has also
resulted in increases in blood pressure (70, 71);
h) High carbohydrate diet may increase significantly the activity of serum lactate dehydrogenase
(149, 150).
i) Lactic acid produced by anaerobic metabolism during cardiac ischemia is among several
compounds suggested to trigger anginal chest pain (13), though the raise in lactate production
has also been recorded in myocardial ischemia without angina pectoris with the distinction
between symptomatic and asymptomatic cases attributed to an individual defect of the stimuli
receptor system and its transformation in painful nervous reflexes in front of equal grade of
ischemia (72);
j) Lactate, acting through extracellular divalent ions, dramatically increases activity of an acidsensing ion channel that is highly expressed on sensory neurons that innervate the heart, being ASIC-3 the most specific to detect ischemic pain (73); 5
k) Lactate accumulation predicts and determines the development and expansion of ischemic
myocardial necrosis (7, 50, 74), albeit cardiotoxic catecholamines may induce myocardial necrosis -- acute contraction band lesions (75);
l) Measurement of arterial blood lactate is considered as a consistently useful prognostic
indicator of survival or fatality in patients with acute myocardial infarction (AMI) and myocardial failure (76);
m) In sudden cardiac death, a histochemical study of enzymatic activity in the myocardium
found that lactate dehydrogenase was 22.6% higher than in trauma and brain hemorrhage that
served as control, seeming to be connected as a response to the catecholamine excess (77).
n) In ischemic stroke acidosis-mediated activation of acid-sensing ion channels may play a role
to ischemic damage of brain tissue (78).
o) Tissue acidosis has been proposed to account for contractile failure during myocardial
ischemia (79, 80) and to contribute for the genesis of cardiac dysrhythmias (81).
It has been demonstrated that pH is lowered intracellularly and extracellularly in ischemic heart
models and clinically in patients with coronary artery disease. In dogs, lowered pH stimulates
afferent cardiac sympathetic nerve fibers. In another organ system, rat skin, acid plays a
dominant role in exciting sensory neurons when compared with other potential chemical
mediators of inflammation (13). In cats, the occlusion of the coronary artery for 5 min decreased
epicardial tissue pH from 7.35 to 6.98 (82).
Acidic environment and atherosclerosis
In advanced plaques the existence of hypoxic areas in the arterial wall – with accumulation of
lactic acid in atherosclerotic lesions – seems related to a decreased oxygen diffusion capacity
and increased oxygen consumption by the foam cells (144).
Macrophages and lymphocytes convert most of their glucose into lactate rather than oxidizing it
completely to CO2, and macrophages possess a selective transporter in their plasma
membranes for lactic acid. This lactic acid may make the extracelullar space surrounding
macrophages acidic in atherosclerotic lesions (83).
A pathological study has demonstrated that approximately two-thirds of the atherosclerotic
plaques show lactate dehydrogenase isoenzyme shifts significantly above that of the media and
intima (84).
It has been reported that lowering pH augments the oxidation of low-density lipoprotein (LDL) by releasing Fe and Cu radicals and decreasing anti-oxidant defense capacity (83, 85, 86).
Recent evidence showed that LDL oxidation occurs not within the interstitial fluid of
atherosclerotic lesions but within lysosomes in macrophages in atherosclerotic lesions. Most
important, the study found that this oxidative modification was inhibited by the drug chloroquine, which increases the pH of lysosomes, as oxidation can be promoted by acidic pH (148).
It has been shown that atherosclerotic plaques have pH heterogeneity, suggesting a possible
role for detecting low pH in the identification of plaque vulnerability. pH heterogeneity can affect numerous plaque functions (87, 88).
Recent in-vitro findings suggest that in areas of atherosclerotic arterial intima, where the
extracellular pH is decreased, binding of apolipoprotein B100 containing lipoproteins to
proteoglycans and modification of the lipoproteins by acidic enzymes are enhanced. The pH
induced amplification of these processes would lead to enhanced extracellular accumulation of
lipoproteins and accelerated progression of the disease (89, 90).
It was suggested that uric acid has antioxidant and prooxidant activities towards the oxidation of native and mildly oxidized LDL, respectively (91). In the atherosclerotic process this antioxidant – prooxidant urate redox shuttle appeared to some investigators to rely heavily on its surrounding environment such as timing (early or late in the disease process), location of the
tissue and substrate, acidity, the surrounding oxidant milieu, depletion of other local
antioxidants, the supply and duration of oxidant substrate and its oxidant enzyme (92).
However, 6 an elevated concentration of lactic acid in blood may inhibit renal excretion of uric acid, leading to its accumulation in the body (145).
Also important is the value of homocysteine and its acidic derivatives as contributors to the
corrosive environment that may lead to the generation of atherosclerosis. Homocysteine, a
sulphur amino acid, is discussed as a cause of atherosclerosis since 1969 (93). It has been
demonstrated that plasma homocysteine levels also increase during psychological stress (94, 95,
96).
It was found that low-density lipoprotein modification is affected by myeloperoxidase (MPO).
MPO’s major product is hypochlorous acid (a weak acid) which appears to be important in the
development process of atherosclerosis. The study has shown that acidic environment play an
important role of hypochlorous acid on LDL modification. A positive correlation was found
between the maximal rate of low-density lipoprotein modification and the acidity of the medium (97, 98, 99). A new study has shown that elevated MPO levels predict future risk of coronary artery disease in apparently healthy individuals. It suggests that inflammatory activation precedes the onset of overt coronary artery disease by many years (100).
It is interesting to notice the evidence that, apart from its occurrence in atherosclerosis, acidity
of the environment is also increased in inflammatory sites (83, 101). This raises a potential
importance of acidity for inflammation results in formation of atheromas.
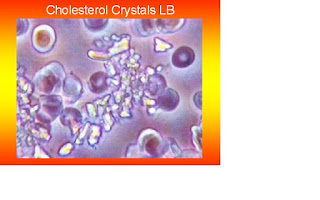
The timing of crystallization depends on several local physical factors, including cholesterol
concentration, pH, temperature, and pressure (102). We believe that the grade in pH of the acidic environment may play a role in the cholesterol crystallization, as it occurs in the formation of uric acid crystals in the development of gout.
Hemodynamic shear stress and atherosclerosis
As the final step in this process the changes in pH may lead to mechanical forces (64, 65, 66, 67) over the acidic coronary blood flow resulted from stress intensifying the damaging action in the development of atherosclerotic lesions.
Atherosclerosis preferentially affects the outer edges of vessel bifurcations. In these
predisposed areas, hemodynamic shear stress, the frictional force acting on the endothelial cell
surface as a result of blood flow is weaker than in protected regions. Studies have identified
hemodynamic shear stress as an important determinant of endothelial function and phenotype
(103).
The pulsatile nature of blood pressure and flow creates hemodynamic stimuli in the forms of
cyclic stretch and shear stress (6).The changes in flow patterns can produce potentially
deleterious effects on vascular biology. Lowered shear stress and oscillatory shear stress are
essential conditions in atherosclerotic lesion size and vulnerability (104, 105). The first paper to
mention about the importance of forces such as those derived from changes in hemodynamic
shear stress could cause atherosclerosis, was wrote by Meyer Texon in 1957 (106, 107).
The plaque rupture
The plaque instability and rupture may start a considerable time before the AMI, according
some studies (108, 109). Stretching, tearing and perforation, related to cholesterol crystallization and expansion, may play a role in the process (102). Also, sympathetic overstimulation and hemodynamic forces like left ventricular muscle mass and elevated heart rate may be associated with the future development of plaque disruption (110).
The release and displacement of thrombi may occur during the acute myocardial infarction (8,
111), being the lactate accumulation (7, 50, 74), regional myocardial insufficiency and stasis of the related artery (7, 111, 112), a plausible mechanism to explain this secondary instability
phenomenon, which may have pancoronary repercussion (113). 7
Some facts supporting the secondary release of coronary thrombus:
a) Increased frequency of thrombi with increasing intervals between onset of the acute
myocardial infarction and death (112);
b) In a significant number of cases angioscopic examination continues to find thrombus on
the presumed culprit lesion, at 6 months after myocardial infarction (114);
c) The frequency of an occlusive thrombus is significantly higher in the larger infarcts (115).
Anyway, the bottom-line is that coronary thrombus is absent in a substantial number of patients as shown in recent studies using intracoronary catheters to aspirate occlusive tissues
performed during myocardial infarction (108, 116). These findings confirm previous autopsy
studies, which came to the conclusion that thrombus are a consequence not a cause of AMI (8,
111, 112).
Stress reduction, sympatholytic agents and regression or lower progression of Atherosclerosis
As a recent paper has shown, coronary atherosclerosis regressed in women who were free of
stress through the use of serial quantitative angiography (117). Its data confirm the results of
other papers. One study reports a decrease in carotid IMT in African Americans with
hypertension submitted to stress reduction through Transcendental Meditation (118). A second
study indicates that a decrease in carotid IMT was related with older persons with multiple
factors for coronary heart disease submitted to the Maharishi Vedic Medicine treatment, which
also includes stress reduction through Transcendental Meditation program (119). A third study
has shown that yoga intervention retards progression and increases regression of coronary
atherosclerosis in patients with severe coronary artery disease (120). Another study has
demonstrated that aerobic physical exercise resulted in statistically significant attenuation in the progression of carotid IMT in middle-aged white men who were not taking statins (121).
Carotid intima-media thickness (IMT) is a valid surrogate measure for coronary atherosclerosis.
Also, studies have shown that rhesus monkeys submitted to sympatholytic agents like
betablockers or bilateral surgical thoracic sympathectomy have had a marked reduction in the
progression of atherosclerosis (122). The first randomized trial showing that betablockers can
reduce the rate of progression of carotid IMT in clinically healthy symptom-free subjects with
carotid plaque was published in 1991 (123) and evidenced later by other studies (124). A recent
pooled analysis data from 4 intravascular ultrasonography trials involving 1,515 patients has
confirmed that betablocker therapy is associated with reduced atheroma progression (125).
To our knowledge only one angiographic study assessed data on regression (15%),
inalterability (62%) or progression (23%) of atherosclerosis in patients treated with cardiac
glycosides (126), that also have sympatholytic properties by blocking excessive release of
catecholamine (48, 49). Years later the same group of researchers from Brazil presented a case
study involving 1,150 patients with coronary-myocardial disease taking daily lower oral doses of
cardiac glycosides – most of times digoxin and digitoxin , showing in a long run (28 years), a
very low mortality rate for cardiac causes, cerebral stroke, cancer or all causes. The global
mortality for patients without previous myocardial infarction was 14,2% (0,5% per year) while for patients with previous myocardial infarction was 41,0% (1,4% per year) (44, 45). Another study, this one made in Germany by Berthold Kern, has achieved similar results. It showed a very low mortality rate in prevention of acute myocardial infarction using sub-lingual cardiac glycoside strophanthin, in about 15,000 patients with heart disease, during 23 years (46).
Implications and Perspectives
A decade ago, the treatment of hypercholesterolemia and hypertension was expected to
eliminate coronary artery disease by the end of the 20th century. Lately, however, that optimistic prediction has needed revision. Cardiovascular diseases are expected to be the main cause of death globally within the next 15 years owing to a rapidly increasing prevalence in developing countries and Eastern Europe and the rising incidence of obesity and diabetes in the Western world (127). This information is contrary to the popular belief that the widespread use of lowering cholesterol drugs like statins could have the potential to become a major effect on the global burden of cardiovascular disease. According to some researchers, statins have been over-hyped and consequently over-used, but not providing significant overall health benefits. Also, that statins have many more side effects than is generally accepted, despite the huge and rising costs (128).
On the other side studies are showing that long term psychological stress is associated with
progression from prehypertension to hypertension or coronary heart disease (129). There are
indications that sympathetic predominance might favour the development of sustained
hypertension and hypercholesterolemia early in life, and lead to increased susceptibility to
vascular complications (130). Also, studies have shown that several components of the
metabolic syndrome, such as obesity and insulin resistance states, are associated with indirect
or direct markers of adrenergic overdrive (131). Yet the elevation of plasma lactate levels may
induce insulin resistance by suppressing glycolysis (132). Moreover, the autonomic nervous
system is influenced by high-carbohydrate dietary, with greater sympathetic nervous activity
(133, 134). Dietary carbohydrate is the major determinant of postprandial glucose levels.
Postprandial hyperglycemia is recognized as a significant risk factor for cardiovascular disease not only in diabetic patients, but also among the general population.
The acid/alkaline theory represents a new paradigm, offering a sea-change in alternatives for the treatment of atherosclerosis, by stress management alone or in adjunct to other pharmaceutical or technological medical approaches. It prioritizes lifestyle modifications like diet, physical exercises, yoga, Transcendental Meditation and through biofeedback stress reduction devices or by other behavioral approaches aimed to reduce chronic stress through relaxation response, consequently decreasing sympathetic bias and its harmful effects.
Accumulated evidence indicates that fish intake and fish oil supplementation reduces
morbidity/mortality associated with cardiovascular disease. In fact, studies are showing that the habitual intake of omega-3 fatty acid may reduce the progression of coronary atherosclerosis (135). Among the possible mechanisms underlying these effects is its capability to reduce the elevated blood lactic acid (136).
Quercetin, the most abundant of the flavonoids, found in high concentration in red wine and in
fruits and vegetables used in Mediterranean diet, may also decrease lactic acid production (137).
This may give an additional explanation on how flavonoids can help to reduce the risk of
atherosclerosis and offer protection against coronary-myocardial disease. Other polyphenols
like resveratrol and curcumin may also reduce lactic acid production in blood (138, 139). Some
phytochemicals with proved therapeutic benefit for the treatment of cardiovascular disease, like
crataegus oxyacantha, have demonstrated in studies a decrease in lactic acid production (140).
Interventions through pharmacological management for atherosclerosis should be used in our
view only in established disease – or in old vulnerable patients, for the restoration of
sympathovagal balance, to slow the progression or in regression of atherosclerosis.
Cardiac glycosides, which are compatible with the acidity theory, should be the drug of choice
for the treatment of atherosclerosis and in prevention of acute coronary syndromes – unstable
angina, myocardial infarction and sudden cardiac death, experienced with success in many
patients with coronary-myocardial disease (44, 45) treated inside the myogenic theory of
myocardial infarction, a complementary hypothesis, where psycho-emotional and physical
stresses are considered to be the main triggers (7, 8).
It was demonstrated in patients taking cardiac glycosides when recovering from myocardial
infarction (141), treated for congestive heart failure (142) or prophylactic in the heart not in failure(143), that a beneficial effect on morbidity and mortality is seen at lower doses but not at higher doses, which are traditionally considered to be therapeutic.
Cardiac glycosides sympatho-inhibitory properties as well other of their important therapeutic
possibilities were confirmed in recent studies. Like re-elevation of lowered pH and to attend the
demand on insufficient production of endogenous DLCs in some clinical conditions.
Due to all of these reasons we believe that the usage of cardiac glycosides may relate to
coronary-myocardial disease the same way as insulin relates to diabetes. 9
It seems that in digitalis therapy, “less is more!†Remembering Paracelsus, 16th century:
“All substances are poisonous; there is none which is not a poison. The right dose
differentiates a poison from a remedyâ€
Original scientific article by Carlos Monteiro "Carlos ETB Monteiro, Acidic environment evoked by chronic stress: A novel mechanism to explain atherogenesis. Available from Infarct Combat Project, January 28, 2008 athttp://www.infarctcombat.org/AcidityTheory.pdf"
Bibliography
1. Pollak O J. 1952, An Etiologic Concept of Atherosclerosis Based on Study of Intimal Alterations after Shock. Circulation;5;539-550. Full free paper at
http://circ.ahajournals.org/cgi/reprint/5/4/539.pdf
2. Ross R, Glomset J, Harker L. 1977. Response to injury and atherogenesis. Am J Pathol. Mar;86(3):675-84
3. Press release. 2006. New Explanation For The Cause Of Atherosclerosis: The Acidity Theory, Medical News Today, Aug 10 at http://www.medicalnewstoday.com/articles/49244.php
4. Press release. 2006. Beyond Lipids: Understanding the Mechanics of Atherosclerosis (press release). UCSD News, July 12. at http://www.jacobsschool.ucsd.edu/news/news_releases/release.sfe?id=554
5. Kaunas R, Usami S, Chien S. 2006 Regulation of stretch-induced JNK activation by stress fiber orientation. Cellular Signalling, Nov;18(11):1924-31 at http://www.ncbi.nlm.nih.gov/pubmed/16581230
6. Haga JH, Li Yi-Shuan J. and Chien S. 2007. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells, Journal of Biomechanics, 40(5):947-60.
7. Mesquita QHde. 1979. Myogenic Theory of Myocardial Infarction (Teoria Miogênica do Enfarte do Miocárdio, Gemini, Sao Paulo, SP - Brazil Book in Portuguese language with a summary in English at http://www.infarctcombat.org/LivroTM/parte8.htm
8. Mesquita QHde, Baptista CAS. 1994. Why Myogenic Theory not Thrombogenic Theory. Arq Bras Cardiol, V. 62 (4) – (Official Journal of Brazilian Cardiology Society). Full translated paper at http://www.infarctcombat.org/MTxTT-ABC.pdf
9. Fernandes VS et al. 2006, Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals. The Multi-Ethnic Study of Atherosclerosis (MESA), J Am Coll Cardiol 47: 2420-8 Full free paper at http://content.onlinejacc.org/cgi/content/full/j.jacc.2005.12.075v1
10. Marwah R, Doux J, Lee P and Yun A. 2007. Is atherosclerosis a neurogenic phenomenon? Medical Hypotheses, V 69, I 4: 884-887
11. Selye H. 1950. The physiology and pathology of exposure to stress: A treatise based on the concepts of the general-adaptation-syndrome and the diseases of adaptationâ€, Montreal, Acta, Inc. / Selye H et al. 1970 Experimental Cardiovascular Diseases, Volume 1 (History, Cardiovascular Disease, Factors Influencing Cardiovascular Disease); Volume 2 (Histology and Histochemistry, Chemical and Functional Changes, References), Springer-Verlag, Berlin New York
12. Cannon WJ. 1914. The emergency function of the adrenal medulla in pain and the major emotions. Am J Physiol. 33:356-372
13. Benson JC, Eckert SP, McCleskey EW. 1999. Acid-Evoked Currents in Cardiac Sensory Neurons – A possible mediator of myocardial ischemic sensation, Circulation Research, 84:921-928. Full free paper at http://circres.ahajournals.org/cgi/content/full/84/8/921
14. Gianni M et al. 2006. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review, European Heart Journal, V27,N13: 1523-1529
15. Akashi YJ et al. 2002. Reversible left ventricular dysfunction "takotsubo" cardiomyopathy related to catecholamine cardiotoxicity, J. Electrocardiol 2002; 35:351-356
16. Arora S et al. 2006. Transient left ventricular apical ballooning after cocaine use; is catecholamine cardiotoxicity the pathologic link? Mayo Clin Proc. 2006; 81:820-832. Full free paper at http://www.mayoclinicproceedings.com/pdf/8106/8106cr2.pdf
17. Wittstein IS et al. 2005. Neurohumoral features of myocardial stunning due to sudden emotional stress, New Engl J Med, Feb 10, V352: 539-548
18. Graham LN, Smith PA et al. 2004. Sympathetic neural hyperactivity and its normalization following unstable angina and acute myocardial infarction, Clin Sci (Lond), Jun;106(6):605-11
19. Gazes PC, Richardson JA et al. 1959. Plasma catecholamine concentrations in myocardial infarction and angina pectoris, Circulation 19:657-661
20. Waldenstrom AP et al. 1978. A possible role of noradrenaline in the development of myocardial infarction, Am Heart J. 95:43-51
21. Nadeau RA, de Champlain J. 1979. Plasma catecholamine in acute myocardial infarction, Am Heart J, 98: 548-554
22. McCance AJ, Thompson PA, Forfar JC. 1993. Increased cardiac sympathetic nervous activity in patients with unstable coronary heart disease, Eur Heart J, Jun;14(6):751-7
23. Makikalio A. 2005. Cardiovascular autonomic and hormonal dysregulation in ischemic stroke with an emphasis on survival, International Journal of Circumpolar Health 64:5
24. Korner P. 2007. Essential Hypertension and Its Causes: Neural and Non-Neural Mechanisms. New York, Oxford University Press
25. Rainforth MV, Schneider RH, Nidich SI, Gaylord-King C, Salerno JW, Anderson JW. 2007. Stress Reduction Programs in Patients with Elevated Blood Pressure: A Systematic Review and Metaanalysis. Curr Hypertens Rep Dec;9(6):520-8. Full free paper at
http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=18350109
26. Barnett PA, Spence JD, Manuck SB, et al. 1997. Psychological stress and the progression of carotid artery disease, J Hypertens 15:49–55
27. Kamarck TW, Everson SA, Kaplan GA, et al. 1997. Exaggerated blood pressure responses during mental stress are associated with enhanced carotid atherosclerosis in middle-aged Finnish men: findings from the Kuopio ischemic heart disease study. Circulation, 96:3842–8 Full free paper at http://circ.ahajournals.org/cgi/content/full/96/11/384210
28. Jennings JR, Kamarck TW et al. 2004. Exaggerated blood pressure responses during mental stress are associated with enhanced carotid atherosclerosis in middle-aged Finnish men: findings from the Kuopio ischemic heart disease study, Circulation;110:2198-2203. Full free paper at http://circ.ahajournals.org/cgi/content/full/110/15/2198
29. Hauss WH et al. 1990. Adrenaline and noradrenaline as possible chemical mediators in the pathogenesis of arteriosclerosis. Ann N Y Acad Sci 598:91-101
30. Matthews KA et al. 1998. Stress-Induced Pulse Pressure Change predicts women’s carotid atherosclerosis, Stroke 29:1525-1530
31. Matthews KA, Zhu S, Tucker DC, Whooley MA. 2006. Blood pressure reactivity to psychological stress and coronary calcification in the Coronary Artery Risk Development in Young Adults Study, Hypertension, Mar; 47(3):391-5. Full free paper at http://hyper.ahajournals.org/cgi/content/full/47/3/391
32. Ghiadone L et al. 2000. Mental stress induces transient endothelial dysfunction in humans, Circulation
102:2473. Full free paper at http://circ.ahajounals.org/cgi/content/full/102/20/2473
33. Steptoe A. et al. 2006. Delayed blood pressure recovery after psychological stress is associated with carotid intima-media thickness. Arterioscler. Thromb. Vasc. Biol. Nov, 26(11):2547-51
34. Eller NH, Netterstrom. 2007. Psychosocial factors at home and at work and four-years progression in intima-media thickness. In J Behav Med 2007; 14 (1):21-29
35. Faramawi et al. 2007. Relation between depressive symptoms and common carotid artery
atherosclerosis in American persons > 65 years of age, Am J Cardiol; 99:1610-1613
36. Schoner W. 2002. Endogenous cardiac glycosides, a new class of steroid hormones. Eur J Biochem. 268,
2440-2448, Full free paper at http://www.ejbiochem.org/cgi/content/full/269/10/2440
37. Nesher M, Shpolansky U, Rosen H, Lichtstein D. 2007.The digitalis-like steroid hormones: New mechanisms of action and biological significance. Life Sci. May 15;80(23):2093-107
38. Sophocleus A et al. 2003. Circulating endogenous digitalis-like factors (EDLF) in man is derived from the adrenals and its secretion is ACTH-dependent. J Endocrinol Invest Jul;26(7):668-74
39. Weidemann H et al. 2004. Diverse effects of stress and additional adrenocorticotropic hormone on digitalislike compounds in normal and nude mice, Journal of Neuroendocrinology, Vol 16, 458-463. Full free paper at http://physiology.huji.ac.il/pdf/lichtstein/weiden-et-al04.pdf
40. Hassan M. AM Qazzaz et al. 2004. De Novo Biosynthesis and Radiolabeling of Mammalian Digitalis-Like Factors. Clin Chem. Mar;50(3):612-20. Full free paper at http://www.clinchem.org/cgi/content/full/50/3/612
41. Rose AM, Valdes RJ. 1994. Understanding the sodium potassium pump and its relevance to disease, Clin. Chem. 40/9: 1674-1685 Full free paper at
http://www.clinchem.org/cgi/reprint/40/9/1674
42. Vasilyev A, Khater K, and Rakowski RF. 2004. Effect of Extracellular pH on Presteady-State and SteadyState Current Mediated by the Na+/K+ Pump,. J Membr Biol. March 15; 198(2):65–76. Full free paper at
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1357233
43. Li C, Geering K, Horisberger JD. 2006. The Third Sodium Binding Site of Na,K-ATPase Is Functionally Linked to Acidic pH-Activated Inward Current. Membr Biol. 213(1):1-9.
44. Mesquita QHde, Baptista CAS. 2002. Cardiotônico: insuperável na preservação da estabilidade miocárdica como preventivo das sÃndromes coronárias agudas e responsável pela prolongada sobrevida, Ars Cvrandi, maio 35:3. Full free paper at http://www.infarctcombat.org/28anos/digitalicos.html . Summary in English at
http://www.infarctcombat.org/heartnews-16.html
45. Mesquita QHde, Baptista CAS et al. 2002. Efeitos do cardiotônico + dilatador coronário na coronáriomiocardiopatia crônica estável, com e sem enfarte prévio, a longo prazo. Ars Cvrandi, setembro;35:7. Full free paper at http://www.infarctcombat.org/qhm/cme.pdf Summary in English athttp://www.infarctcombat.org/heartnews-16.html
46. Kern B. 1970. Der Myokard-Infarkt. Haug-Verlag, Heidelberg.
47. Gao JRS et al. 2002. Isoform specific stimulation of cardiac Na/K pumps by nM concentrations of glycosides, J Gen Physiol 119:297-312. Full free paper at http://www.jgp.org/cgi/content/full/119/4/297
48. Schobel HP et al. 1991.Contrasting effects of digitalis and dobutamine on baroreflex sympathetic control in normal humans, Circulation V84, 1118-1129. Full free paper at http://circ.ahajournals.org/cgi/reprint/84/3/1118
49. Gutman Y, Boonyaviroj P. Naunyn Schmiedebergs. 1977. Mechanism of inhibition of catecholamine release from adrenal medulla by diphenylhydantoin and by low concentration of ouabain (10 (-10) M). Arch Pharmacol Feb;296(3):293-6
50. von Ardenne M. 1978. Die Hemmung der mikrozirculation beim myokardinfarkt und das perlingual applizierte g-strophanthin, Arzneimittel-Forsch. 28; 202:
51. Pierre SV et al. 2007. Ouabain triggers preconditioning through activation of the NA+, K+-ATPase signalling cascade in rat hearts, Cardiovasc Res, Feb 1;73(3): 488-96
52. Pugin J, Dunn-Siegrist I, Dufour J, Tissieres P, Charles PE, Comte R. 2007. Cyclic Stretch of Human Lung Cells Induces an Acidification and Promotes Bacterial Growth, Am J Respir Cell Mol Biol. Oct 5 doi:10.1165/rcmb.2007-0114OC
53. Levy B, Gibot S, Franck P, Cravoisy A, Bollaert PE. 2005. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet. Mar 5-11;365(9462):871-5.
54. Schade DS.1982. The role of catecholamines in metabolic acidosis. Ciba Found Symp;87:235-53
55. Abarquez RF Jr. 1967. Digitalis in the treatment of hypertension. A preliminary report. Acta Med Philipp. Jan-Mar;3(3):161-70
56. Yuan CM, Manunta P, Hamlyn JM et al. 1993. Long-term ouabain administration produces
hypertension in rats. Hypertension, 3;22;178-187 Full free paper at http://hyper.ahajournals.org/cgi/reprint/22/2/178
57. Manunta, P., Hamilton, J., Rogowski, A.C., Hamilton, B.P., Hamlyn, J.M. 2000. Chronic hypertension induced by ouabain but not digoxin in the rat:antihypertensive effect of digoxin and digitoxin. Hypertension Research 23 (Suppl), S77–S85.
58. Yang Q, Huang W, Jozwik C, Lin Y, Glasman M et al. 2005. Cardiac glycosides inhibit TNF-alpha/NF-kappaB signaling by blocking recruitment of TNF receptor-associated death domain to the TNF receptor. Proc Natl Acad Sci USA Jul 5;102(27):9631-6. Full free paper at http://www.pnas.org/cgi/content/full/102/27/963111
59. Sternberg EM. 2001. Neuroendocrine regulation of autoimmune/inflammatory disease, J Endocrinol Jun; 169(3):429-35. Full free paper at http://joe.endocrinology-journals.org/cgi/reprint/169/3/429
60. Brum PC, Kosek J, Patterson A et al. 2002. Abnormal cardiac function associated with sympathetic nervous system hyperactivity in mice. Am J Physiol Heart Circ Physiol 283: H1838-H1845. Full free paper at http://ajpheart.physiology.org/cgi/content/full/283/5/H1838#B4
61. F. E. Demartini, P. J. Cannon, W. B. Stason, and J. H. Laragh. 1965. Lactic Acid Metabolism in Hypertensive Patients. Science 11 June, Vol. 148. no. 3676, pp. 1482 – 1484
62. Sharda S, Gupta SN and Khuteta KP. 1975. Effect on mental stress on intermediate carbohydrate-and lipidmetabolism. Indian J Physiol Pharmacol. Apr-Jun;19(2):86-9.
63. Hall JB, Brown DA. 1979. Plasma glucose and lactic acid alterations in response to a stressful exam. Biol Psychol. May;8(3):179-88.
64. von Ardenne M, Reitnauer PG. 1989. Increase of perfusion pressure at constant perfusion rate caused by low pH values, Biomed Biochim Acta, 48(4):317-23
65. Yasushi Horai et al. 2005. Changes in pH increase perfusion pressure of coronary arteries in the rat. J Pharmacol Sci 97; 400: 407
66. Austin C, Wray S. 2000. Interactions Between Ca2+ and H+ and Functional Consequences in Vascular Smooth Muscle, Mini Review, Circulation Research 86:355. Full free paper at
http://circres.ahajournals.org/cgi/content/full/86/3/355
67. Kim YM et al. 2005. Contribution of Na_-K_ pump and KIR currents to extracellular pH-dependent changes of contractility in rat superior mesenteric artery, Am J Physiol Heart Circ Physiol 289:792-800 Full free paper at http://ajpheart.physiology.org/cgi/reprint/289/2/H792
68. Carter G, Gavin JB. 1989. Endocardial damage induced by lactate, lowered pH and lactic acid in nonischemic beating hearts. Pathology Apr;21(2):125-30
69. Sharma AM, Kribben A et al. 1990. Salt sensitivity in humans is associated with abnormal acid-base balance. Hypertension; 16, 407-413. Full free paper at http://hyper.ahajournals.org/cgi/reprint/16/4/407
70. Harold T. Edwards, Edward H. Bensley, David B. Dill and Thorne M. Carpenter. 1944. Human Respiratory Quotients in Relation to Alveolar Carbon Dioxide and Blood Lactic Acid After Ingestion of Glucose, Fructose, or Galactose. Journal of Nutrition Vol. 27 No. 3 March, pp. 241-251. Full free paper at http://jn.nutrition.org/cgi/reprint/27/3/241
71. Hallfrisch J. 1990. Metabolic effects of dietary fructose. FASEB J, Vol 4; Jun: 2652-2660. Full free paper at http://www.fasebj.org/cgi/reprint/4/9/2652.pdf
72. Mesquita QHde. 1982. Aspectos angiográficos coronários e ventriculograficos do primeiro enfarte do miocárdio em coronariopatia crônica silenciosa. Rev. Bras. Med., V 39: N7
73. LA Naves and McCleskey EW. 2005. An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res, 38 (11) 1561-69 http://www.scielo.br/pdf/bjmbr/v38n11/v38n11a01.pdf
74. Vogt AM, Ackermann C, Yildiz M, Schoels W, Kübler W. 2002. Lactate accumulation rather than ATP depletion predicts ischemic myocardial necrosis: implications for the development of lethal myocardial injury, Biochim Biophys Acta Mar 16;1586(2):219- 26.
75. Todd GL, Baroldi G, Pieper GM, Clayton FC, Eliot RS. 1985. Experimental catecholamine- induced myocardial necrosis. I. Morphology, quantification and regional distribution of acute contraction band lesions. J Mol Cell Cardiol. Apr 17(4):317- 38.
76. Henning RJ, Well MH, Weiner F. 1982. Blood lactate as prognostic indicator of survival in patients with acute myocardial infarction. Circ Shock, 9(3):307-15
77. Vikhert AM, Cherpachenko NM. 1985. Histoenzymological characteristics of the myocardium in sudden cardiac death. Arkh Patol 47(7):29-34
78. Huang Y, McNamara JO. 2004. "Ischemic Stroke: “Acidotoxicity†Is a Perpetrator", Cell, Volume 118, Issue 6, 17 September, Pages 665-666
79. Tennant R. 1935. Factors concerned in the arrest of contraction in an ischemic myocardial area. Am J Physiol: 133; 677-682
80. Katz AM, Hecht H. H. 1969. The early pump failure of the ischaemic heart. Am J Med: 47; 497-502
81. Elharrer V, Zipes D.P. 1977. Cardiac electrophysiologic alterations during myocardial ischaemia. Am J Physiol: 233: H329-345
82. Pan HL et al. 1999. Role of protons in activation of cardiac sympathetic C-fibre afferents during ischaemia in cats. J Physiol. Aug 1;518 ( Pt 3):857-66. Full free paper at http://jp.physoc.org/cgi/content/full/518/3/857
83. Leake DS. 1997. Does an acidic pH explain why low density lipoprotein is oxidised in atherosclerotic lesions? Atherosclerosis. Mar 21;129(2):149- 57
84. Gown MA, Benditt PE. 1982. Lactate dehydrogenase (LDH) isozymes of human atherosclerotic plaques. Am J Pathol 1982, 107:316-321
85. Morgan J, Leake DS. 1995. Oxidation of low density lipoprotein by iron or copper at acidic pH. J Lipid Res. Dec;36(12):2504- 12. Full free paper at
http://www.jlr.org/cgi/reprint/36/12/2504
86. Patterson RA, Leake DS. 1998. Human serum, cysteine and histidine inhibit the oxidation of low density lipoprotein less at acidic pH. FEBS Lett. Sep 4;434(3):317- 21.
87. Naghavi M et al. 2002. pH Heterogeneity of human and rabbit atherosclerotic plaques; a new insight into detection of vulnerable plaque. Atherosclerosis Sep, V 164; 1:27-35
88. Khan T, Soller B, Naghavi M, Casscells W. 2005. Tissue pH determination for the detection of metabolically active inflamed vulnerable plaques using near-infrared spectroscopy: an in-vitro feasibility study. Cardiology.; 103(1): 10-6.
89. Sneck M, Kovanen PT, Oorni K. 2005. Decrease in pH strongly enhances binding of native, proteolysed, lipolysed, and oxidized low density lipoprotein particles to human aortic proteoglycans, Journal of Biological Chemistry, 280;45: Nov. Full free paper at http://www.jbc.org/cgi/reprint/280/45/37449
90. Oorni K and Kovanen PT. 2006. Enhanced extracellular lipid accumulation in acidic environments. Curr Opin Lipidol 17(5);534-40: Oct
91. Patterson RA, Horsley ETM, Leake DS. 2003. Prooxidant and antioxidant properties of human serum ultrafiltrates toward LDL: important role of uric acid. Journal of Lipid Research, Vol. 44, 512-521, March Full free paper at http://www.jlr.org/cgi/reprint/44/3/51212
92. Hayden MR, Tyagi SC. 2004. Uric acid: A new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: The urate redox shuttle. Nutr Metab (Lond). 1: 10, October 19. Full paper at http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=529248
93. McCully KS. 1969. Vascular pathology of homocysteinemia: implications for the pathogenesis of atherosclerosis. Am J Pathology 56:111:28
94. Stoney CM. 1999. Plasma homocysteine levels increase in women during psychological stress, Life Sci 64(25):2359-65
95. Stoney CM and Engebretson TO. 2000. Plasma homocysteine concentrations are positively associated with hostility and anger, Life Sci 66(23):2267-75
96. Hapuarachchi JR, Chalmers AH et al. 2003. Changes in clinically relevant metabolites with psychological stress parameters. Behav Med. Summer;29(2):52-9
97. Jerlich A et al. 1999. Correlation of low-density lipoprotein modification by myeloperoxidase with hypocholorous acid formation, Int. J. Clin, Lab, Res 29(4):155-61
98. Podrez EA, Abu-Soud HM, Hasen SL. 2000. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med 28:1717–1725
99. Yang J, Cheng Y, Ji R, Zhang C. 2006. Novel model of inflammatory neointima formation reveals a potential role of myeloperoxidase in neointimal hyperplasia. Am J Physiol Heart Circ Physiol. Dec;291(6):H3087- 93.
100. Meuwese MC, Stroes ESG, Hazen SL, et al. 2007. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: The EPIC-Norfolk Prospective Population Study..J Am Coll Cardiol 50:159-165
101. Wong ML et al. 2000. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: A possible link between inflammatory cytokines and atherogenesis, PNAS 97;8681-8686 Full free paper at http://www.pnas.org/cgi/content/full/97/15/8681
102. Abela GS. 2006. Plaque Rupture by Cholesterol Crystallization - A Novel Concept for Acute Coronary Syndrome, American College of Cardiology Annual Scientific Session, March 13, Full free paper at http://www.cardiosource.com/rapidnewssummaries/summary.asp?SumID=164
103. Malek AM, Alper SL, Izumo S. 1999. Hemodynamic shear stress and its role in atherosclerosis JAMA 282: 2035-2042
104. Cheng C et al. 2006. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113:2744-2753. Full free paper at at
http://circ.ahajournals.org/cgi/content/abstract/113/23/2744
105. Cunningham KS and Gotlieb AI. 2005. The role of shear stress in the pathogenesis of atherosclerosis (Mini review), Laboratory Investigation 85, 9-23, Full free paper at
http://www.nature.com/labinvest/journal/v85/n1/full/3700215a.html
106. Texon M. 1957. A hemodynamic concept of atherosclerosis, with particular reference to coronary occlusion. Arch Intern Med 99:418–427
107. Imparato AM, Lord JW Jr, Texon M, Helpern M. 1961. Experimental atherosclerosis produced by alteration of blood vessel configuration. Surg Forum 12:245–247.
108. Rittersma SZH, van der Wal AC, Koch KT, et al. 2005. Plaque instability frequently occurs days or weeks before occlusive coronary thrombosis. A pathological thrombectomy study in primary percutaneous coronary intervention. Circulation; 111:1160-1165. Full free paper at
http://circ.ahajournals.org/cgi/content/full/111/9/1160
109. Ojio S, Takatsy H, et al. 2000. Considerable time from the onset of plaque rupture and/or thrombi until the onset of acute myocardial infarction in humans coronary angiographic findings within 1 week before the onset of infarction. Circulation;102:2063. Full free paper at
http://www.circ.ahajournals.org/cgi/reprint/102/17/2063
110. Ulrich E. Heidlan, Bodo E. Strauer. 2001. Left ventricular muscle mass and elevated heart rate are associated with coronary plaque disruption, Circulation 104:1477. Full free paper at
http://circ.ahajournals.org/cgi/content/full/104/13/1477
111. Baroldi G, Bigi R, Cortigiani L. 2004. Ultrasound imaging versus morphopathology in cardiovascular diseases. Coronary collateral circulation and atherosclerotic plaque. Cardiovascular ultrasound; 3: 6. Full free paper at http://www.cardiovascularultrasound.com/content/3/1/6
112. Roberts W. C. 1974. Coronary Thrombosis and Fatal Myocardial Ischemia. Circulation;49;1-3 Full free paper at http://circ.ahajournals.org/cgi/reprint/49/1/1.pdf
113. Rioufol G, Finet G, Andre-Fouet X et al. 2002. Multiple atherosclerotic plaque rupture in acute coronary syndrome: a three-vessel intravascular ultrasound study. Circulation; 106:804-808. Full free paper at http://www.circ.ahajournals.org/cgi/reprint/01.CIR.0000025609.13806.31v1
114. Yasunori Ueda, Masanori Asakura, et al. 2001. The healing process of infarct-related plaque: Insights from 18 months of serial angioscopic follow-up. Am Coll Cardiol, 38:1916-1922.
Full free paper at http://content.onlinejacc.org/cgi/reprint/38/7/1916
115. Giorgio Baroldi, Riccardo Bigi and Lauro Cortigiani.2005. Ultrasound imaging versus
morphopathology in cardiovascular diseases. Myocardial cell damage. Cardiovascular Ultrasound 3:32. Full free paper at http://www.cardiovascularultrasound.com/content/3/1/32
116. Murakami T, Mizuno S, Takahashi Y, Ohsato K et al. 1998. Intracoronary aspiration thrombectomy for acute myocardial infarction, Am. J Cardiology Oct 1;82 (7):839-44
117. Wang HX, Leineweber C, et al. 2007. Psychosocial stress and atherosclerosis: family and work stress accelerate progression of coronary disease in women. The Stockholm Female Coronary Angiography Study. Journal of Internal Medicine 261;245-254
118. Richmond AC et al. 2000. Effects of stress reduction on carotid atherosclerosis in hypertensive African Americans, Stroke 31:568-573. Full free paper at http://stroke.ahajournals.org/cgi/reprint/31/3/568
119. Fields JZ et al. 2002. Effect of a multimodality natural medicine program on carotid atherosclerosis in older subjects: a pilot trial of Maharishi Vedic Medicine, American Journal of Cardiology, 89; 8:952-958
120. Manchanda SC, Narang R, Reddy KS, Sachdeva U, Prabhakaran D, Dharmanand S, Rajani M and Bijlani R. 2002. Retardation of coronary atherosclerosis with yoga lifestyle prevention, J Assoc Physicians India Jul; 48(7): 687-94 13
121. Rainer Rauramaa et al. 2004. Effects of aerobical physical exercise on inflammation and atherosclerosis in men: The DNASCO Study. Annals of Internal Medicine, 15 June, 140:12:1007-1014
122. Lichtor T et al. 1987.The sympathetic nervous system and atherosclerosis. J Neurosurg Dec;67(6):906-14
123. Pauletto P et al. 1991. Sympathetic drive and vascular damage in hypertension and atherosclerosis, Hypertension Apr;17(4 Suppl):III75-81
124. Wikstrand J, Berglund G, Hedblad B, Hulthe, Wikstrand J. 2003.Anti-atherosclerotic effects of beta-blockers.
Am J Cardiol. Jun 19;91(12A):25H-29H.
125. Sipahi I et al. 2007. B-Blockers and progression of coronary atherosclerosis; Pooled analysis of 4 intravascular trials. Annals of Internal Medicine, 3 July, V147; Issue 1: 10-18
126. Mesquita QHde, Kerbrie SV, Mari SM, Baptista CA, Monteiro J, Maciel MC. 1978. Preservação funcional do miocárdio isquêmico pelo cardiotonico a longo prazo: recateterização de 29 casos. Medicina de Hoje, março 1978
127. Hansson GK. 2005. Inflammation, atherosclerosis and coronary artery disease, NEJM V 352; N16 April 21
128. Malcolm Kendrick. 2007. Are statins overused? Future Lipidol, 2 (5)
129. Player MS, King DE, et al. 2007. Psychosocial Factors and Progression From Prehypertension to Hypertension or Coronary Heart Disease, Ann Fam Med ;5(5):403-411. Full free paper at http://www.medscape.com/viewarticle/565806?src=mp
130. Palatini P, Longo D, Zaetta V, Perkovic D, Garbelotto R, Pessina AC. 2006. Evolution of blood pressure and cholesterol in stage 1 hypertension: role of autonomic nervous system activity, J Hypertens.Jul;24(7):1375-81.
131. Grassi G, Quarti-Trevano F, Seravalle G, Dell’Oro R. 2007. Cardiovascular risk and adrenergic overdrive in the metabolic syndrome. Nutr Metab Cardiovasc Dis Jul; 17(6): 473-81
132. Choi CS, Kiim YB, Lee FN, et al. 2002. Lactate induces insulin resistance in skeletal muscle by suppressing glycolysis and impairing insulin signaling. Am J Physiol Endocrinol Metab 283: E233–E240, 2002. Full free paper at http://ajpendo.physiology.org/cgi/content/full/283/2/E233
133. Tentolouris N, Tsigos C, Perea D et al. 2003. Differential effects of high-fat and high-carbohydrate isoenergetic meals on cardiac autonomic nervous system activity in lean and obese women. Metabolism. Nov;52(11):1426- 32
134. Calynn Davis Bunol, 2005. Thesis, Autonomic nervous system modulation of the heart following a high carbohydrate liquid meal, December. Full free paper at http://etd.lsu.edu/docs/available/etd-09082005-165133/unrestricted/Bunol_thesis.pdf
135. Erkilla AT, Matthan NR, et al. 2006. Higher plasma docosahexaenoic acid is associated with reduced progression of coronary atherosclerosis in women with CAD. J Lipid Res; 47: 2814-19 Full free paper at http://www.jlr.org/cgi/reprint/47/12/2814
136. Ogilve GK, Fettman MJ et al. 2000. Effect of fish oil, arginine, and doxorubicin chemotherapy on remission and survival time for dogs with lymphoma: A double-blind, randomized placebo-controlled study, Cancer; 88: 1016-28. Full free paper at http://www3.interscience.wiley.com/cgibin/fulltext/75504731/PDFSTART
137. Graziani Y. 1977. Regulation of cyclic AMP level and lactic acid production in Ehrlich ascites tumor cells. Biochim Biophys Acta April 27;497(2):499-506
138. Nazam Ansari N, Bhandari U, Pillai KK. 2007. Protective role of curcumin in myocardial oxidative damage induced by isoproterenol in rats. Hum Exp Toxicol, Dec;26(12):933-8
139. Dernek S et al. 2004. Cardioprotection with resveratrol pretreatment: improved beneficial effects over standard treatment in heart rats after global ischemia. Scand Cardiovasc J Aug;38(4):245-54
140. Al Makdessi S, Sweidan H, Müllner S, Jacob R. 1996. Myocardial protection by pretreatment with Crataegus oxyacantha: an assessment by means of the release of lactate dehydrogenase by the ischemic and reperfused Langendorff heart. Arzneimittelforschung Jan;46(1):25-7.
141. Leor J, Goldbourt U et al. 1995. Digoxin and increased mortality among patients recovering from acute myocardial infarction: importance of digoxin dose, Cardiovasc Drugs Ther. Oct;9(5):723-
142. Adams KF Jr, Patterson JH et al. 2005. Relationship of serum digoxin concentration to mortality and morbidity in women in the digitalis investigation group trial: a retrospective analysis. J Am Coll Cardiol. Aug 2;46(3):497-504
143. Wycoff C.C. 1969. New Concepts of Digitalis, Calif Med. 1969 December; 111(6): 423–432. Full free paper at http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1503737
144. T Bjornheden, M Levin, M Evaldsson, O Wiklund. 1999. Evidence of hypoxic areas within the arterial wall in vivo, Arteriosclerosis, Thrombosis and Vascular Biology; 19:870-876
145. Quick AJ. 1935. The effect of exercise on the excretion of uric acid. The Journal of Biological Chemistry. Full free paper at http://www.jbc.org/cgi/reprint/110/1/107.pdf
146. Flierl MA, Rittirsch D, Nadeau BA et al. 2007. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature Oct 11;449 (7163):721-5
147. Lee KS and Klaus W. 1971. The subcellular basis for the mechanisms of inotropic action of cardiac glycosides. Pharmacol Rev 23:193-261
148. Wen Y, Leake DS. 2007. Low density Lipoprotein oxidation undergoes within lysosome in cells. Circ.Res. 100;1337-1343. Full free paper at http://circres.ahajournals.org/cgi/content/full/100/9/1337
149. Marshall MW and Iacono JM (1976). Changes in lactate dehydrogenase, LDH isoenzymes, lactate, and pyruvate as a result of feeding low fat diets to healthy men and women. Metabolism. 1976 Feb;25(2):169-78.
150. Yoshimura T, Miyoshi T, et al. (1986). Effect of high carbohydrate diet on serum lactate
dehydrogenase isozyme pattern in Japanese young men. Acta Biol Hung. 1986;37(3-4):243-8.






0 nhận xét:
Đăng nhận xét